A cikin ilimin sunadarai, akwai abubuwa da yawa waɗanda ƙila ko ba su da alaƙa da juna. Akwai karafa, nonmetals, lanthanides da actinides, ƙananan ƙarfe da ƙananan alkaline; kuma tabbas muna da daya daga cikin sinadarai Wanda ba mu ba da kulawa sosai ba a yayin karatun ilmin sunadarai, kuma mafi ƙarancin abin da ya shafi ci gaba da rayuwarmu. Ina magana ne, ba shakka, game da iskar gas masu daraja.
Waɗannan abubuwa waɗanda, kasancewar suna da ƙarancin yanayi, ba zamu iya yin bincike da yawa ba. Anan za mu koya game da tarihin gas mai daraja, amfaninsu da dukiyoyinsu, da sauran abubuwan sha'awa. Tsaya anan ka koyi abubuwa mafi kyau game da iskan gas.
Bari mu san gas
Areungiyar mahaɗan sunadarai ne masu kamanceceniya da juna. Misali, a karkashin yanayi na yau da kullun basu da launi, basu da kamshi, ba su da isasshen iska, kuma suna da karfin tasirin sinadarai sosai. Waɗannan suna cikin rukunin lamba 18 na tebur na lokaci-lokaci, kuma an san su da: Helium, neon, Xenon, Argon, Krypton, radiyo: Radon, da na roba: Oganeson.
Za'a iya bayanin kaddarorin ta da ra'ayoyin zamani game da kwayar zarra. Kwancen su na lantarki masu ɗauka ana ɗaukarsu cikakke, wanda ke basu ƙarancin halin shiga cikin halayen sunadarai, kuma yana ɗaya daga cikin dalilan da yasa aka kasa fahimtarsu. A zahiri, kaɗan ne daga cikin mahaɗan iskar gas aka shirya har zuwa yau.
A ina muke samun iskan gas?
Muna samun neon, argon, xenon da krypton daga iska ta amfani da hanyoyin rarrabuwa da liquefaction. Ana samun sinadarin helium a cikin iskar gas, inda ya kamata yawanci ya rabu. Kuma radon yana samuwa ta hanyar lalacewar radiyo na mahadi da aka narkar a cikin radium.
Kuma Oganeson wani abu ne na roba da aka kirkira a shekarar 2002, kuma wanda ya samo sunansa na IUPAC a shekarar 2016. An san shi da kasancewa mai amsawa sosai da kuma rashin kwanciyar hankali, saboda haka ba a yi aiki mai yawa da shi ba.
Wadannan gas din sunada matukar amfani a fagen haske, walda da kuma binciken sararin samaniya. Trimix, wanda shine maganin helium-oxygen-nitrogen, ana amfani dashi ta yadda masu saɓo ba zasu sha wahala tasirin narkewar nitrogen a cikin zurfin ba. Menene ƙari, bayan sanin haɗarin haɗarin hydrogen, wannan an maye gurbinsa da helium wajen ƙirƙirar iska da kuma balon iska mai zafi.
Kadarorin waɗannan gas
Gas masu daraja sunaye sunansu daga fassarar daga Jamusanci edelgas, sunan da aka yi amfani da shi a karo na farko a cikin 1898 daga masanin sunadarai Hugo Erdman. Da wannan sunan ya nemi komawa zuwa ƙimar reactivity daga cikin wadannan abubuwan. A zahiri, waɗannan sune mafi ƙarancin abubuwan da aka sani, saboda haka kusan basa aiki ko basa aiki.
Wannan saboda suna da cikakken harsashi na valence wanda ya bar su da ƙaramar damar sakin electrons kuma yana sanya halayensu kusa da na kyakkyawan gas.
Gabaɗaya, gas masu daraja suna da halaye daban-daban.
- Abubuwa ne marasa ƙarfe: Kasancewarka gas, bashi da wata kwayar ƙarfe a cikin yanayin yadda yake. A lokaci guda ba za su iya amsa tare da sauran ƙarfe ba.
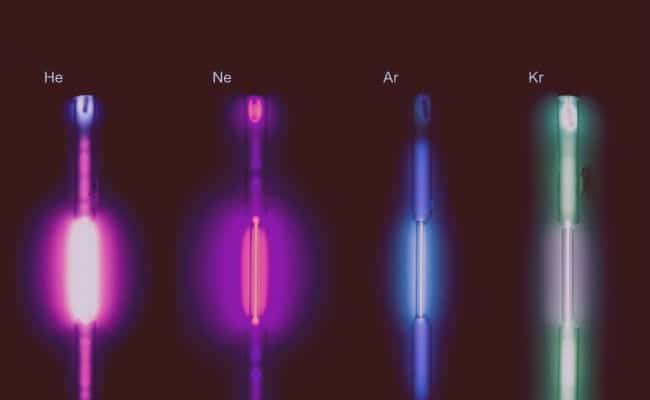
- Ba su da launi kuma ba su da ƙamshi: ko da yake ana iya ba su launuka zuwa kwararan fitila da fitilu an halicce ta ta wadannan gas din ta amfani da wutar lantarki, asali basu da launi, kuma basu da wari.
- Suna da cikakken rufin valence: Neon, xenon, argon, krypton, da kuma radon suna da lantarki guda takwas a cikin kwanansu na karshe. A nasa bangaren, helium yana da lantarki guda biyu. Ta wannan hanyar, iskar gas masu daraja suna da cikakkiyar kwalliya. Wannan shine dalilin da ya sa, a cikin yanayi na yau da kullun, waɗannan abubuwan ba sa yin mahaɗi.
- Suna wanzu a matsayin gas na monatomic: Kamar yadda aka fahimta, waɗannan abubuwan, har ma da mafi girman atomatik, suna da atom ɗaya ne kawai.
- Ba su da tasiri sosai: Saboda cikakkiyar ƙarfinsu da wahalar isar da wutan lantarki, ana ɗaukarsu kamar basa aiki.
- Suna gudanar da wutar lantarki kuma suna samar da haske: Kodayake suna da rauni sosai, waɗannan gas suna da ikon gudanar da wutar lantarki, kuma a yin hakan suna haskakawa da ruwa.
- Suna da ƙaramin narkewa da tafasar ruwaWaɗannan gas ɗin masu daraja suna da ƙananan narkewa da wuraren dahuwa.
- Bã su da wani sosai low electronegativity: wadannan abubuwan yanada karancin wutan lantarki
- Bã su da wani babban ionization makamashi: Arfin ku na ionization shine ainihin mafi girma a cikin lokacin ku.
- Ba su da wuta: Ko da saboda wuta mai saurin kunnawa, an maye gurbinsa da sinadarin helium wajen kera iska da balan-balan.
Kamar yadda yake tare da amsawa, ƙarfin haɗin gwiwar su ma suna da rauni sosai, wanda shine dalilin da yasa suke da ƙananan narkewa da tafasasshen yanayin zafi, kuma dukkansu gas ɗin monatomic ne a ƙarƙashin yanayi na yau da kullun, gami da iskar gas mai ƙarfin atom.
Helium yana da kaddarorin da yawa waɗanda babu wani kyakkyawan gas ko wani abu akan tebur na lokaci-lokaci. Nasa Matsayin narkewa shine mafi ƙasƙanci a cikin dukkan sanannun, ban da kasancewa kawai element wanda ya mallaki yanayin superfluidity; - yanayin da kwayar halitta take cikin yanayin ruwa, amma zai iya gudana ba tare da rasa kuzarin kuzari ba. Helium yana buƙatar matsi na 25atm da zazzabi na -272ºC don samun damar ƙarfafawa.
Cikakken kwandon su yana da alhakin waɗannan gas ɗin da ke da ƙarfin ionization (mafi girma a cikin tebur na lokaci-lokaci). kuma ba zai iya samar da ions cikin sauki ba, wanda ke nuna kwanciyar hankali a cikin saitunan lantarki.
Arfin ionization yana raguwa yayin da ƙungiyar ke raguwa, tunda radius atomic yana ƙaruwa kuma electrons valence suna nesa da tsakiya don haka basu da sha'awar shi. Wannan yana haifar da cewa, kodayake lokacinta shine mafi girma, wasu gas masu daraja suna da kwatankwacin ionization makamashi zuwa wancan na sauran abubuwa. Misali, ionization kuzari na xenon yana kama da ionization kuzari na oxygen.
Amfani da waɗannan gas
Ta hanyar samun ƙananan ƙananan tafasa da wuraren narkewa, suna da amfani musamman wajen kera kayan aikin sanyaya daki, kuma yana sanya su masu amfani azaman masu sanyaya kaya masu kyau.
Ana amfani da sinadarin helium mai ruwa, wanda ya tafasa da 4,2K (-268,93ºC) wajen kera manyan maganadiso, kamar wadanda aka yi amfani dasu don hoton magnetic resonance da nukiliyar maganadisu.
Neon na ruwa, kodayake bai kai yanayin zafi na helium mai ruwa ba, yana da ƙarin aikace-aikace a cikin kayan mai, tunda yana da ƙarfin 40 sau fiye da sanyaya fiye da helium ruwa kuma ya ninka na hydrogen ruwa sau 3.
Ana amfani da sinadarin helium a matsayin wani bangare na iskar gas mai iya numfashi don maye gurbin nitrogen, saboda shi low solubility cikin ruwaye, musamman a cikin kitse. Gaz yana shiga cikin jini da kyallen takarda lokacin da ake samun matsin lamba, kamar su ruwa, wanda ke haifar da sakamako mai sa maye wanda ake kira zurfin cuta. Saboda ƙarancin solubility, ƙaramin helium yana shiga membranes ɗin salula, wanda ke taimakawa wajen magance tasirin narcotic.
Saboda karancin kuzari da haske, kuma bayan bala'in Hindenburg na 1937, helium ya maye gurbin sinadarin hydrogen wajen kera mai, duk da asarar buoyancy na 8,6%
Ana amfani da waɗannan gas ɗin a cikin haske saboda tasirin su. A yayin kera kwararan fitila ana amfani da cakuda argon da nitrogen don cika su. Krypton amfani da babban kwararan fitilaKamar fitilun halogen, waɗanda suke da tsananin zafin launi da inganci.
Xenon ana amfani dashi a cikin fitilun xenon, wanda, ta hanyar samun haske na haske kama da hasken rana, ana amfani dashi a cikin masu shirya fim da kuma fitilar mota.
A cikin magani, ana amfani da helium don inganta sauƙin numfashi a cikin marasa lafiyar asma. Ana iya amfani da Xenon azaman maganin sa maye Saboda yawan solubility a cikin lipids, wanda ya sa ya zama mafi tasiri fiye da yadda ake amfani da shi, kuma kamar yadda jiki yake kawar da shi, yana ba da damar saurin dawowa.
Samun hotunan da ake aiwatarwa ta hanyar tasirin maganadisu na nukiliya, yana da xenon haɗe da sauran gas. Radon, wanda ke da tasirin rediyo sosai kuma ana samun sa ne kawai a cikin adadi kaɗan, ana amfani dashi a cikin maganin kulawar radiation.
Production da yalwa
Yawa da sauƙi wanda za'a iya samun iskar gas masu kyau suna cikin akasi daidai da lambar atomatik. Sabili da haka, yawan waɗannan gas ɗin yana raguwa yayin da lambar atomatik suke ƙaruwa.
A cikin duniya, helium shine abu mafi sauki na biyu da za'a samu, bayan hydrogen, da kusan kashi 24%. Mafi yawan adadin helium a sararin samaniya an kirkireshi ne ta hanyar nucleusynthesis na farko, amma yawansa yana karuwa ne saboda sa hannun hydrogen a cikin tauraron dan adam (wani tsari wanda yake fitowa daga halayen nukiliya yayin aiwatarwar juyin halitta na taurari).
Sauran gas basu kusan yawaita ko sauƙin samu ba. Radon, alal misali, na iya zama tsari a cikin lithosphere ta hanyar lalacewar alpha na radium; A halin yanzu shi xenon ya inganta ka'idar da ake kira "bacewar xenon" saboda karancin adadin shi a sararin samaniya.
Bari muyi magana kadan game da kowane
- Helio: Saboda karancin abin da yake iya amfani da shi, kuma saboda shi ne abu mafi sauki na biyu da ake samu, ya sami damar maye gurbin sinadarin hydrogen a matsayin abin da zai iya cika balloons da zeppelins, tunda basa fashewa lokacin da suka hadu da wuta.
- Neon: Wannan gas din, saboda haske da kuma launin ja-lemu da aka samu lokacin da ya sadu da wutar lantarki, ana amfani dashi don dalilai na talla. An samo shi a sauƙaƙe a cikin hasken neon. Hakanan ana samun tubunan Neon da fitilun da suke da wasu launuka, kodayake suna da sauran gas a ciki.
- Argon: Ana amfani da wannan gas ɗin a cikin fitilun da ke haskakawa saboda baya yin aiki tare da filament ɗin a ƙarƙashin babban yanayin zafin jiki da matsin lamba. A cikin tubes mai kyalli yana haifar da launi mai launin shuɗi-shuɗi. Hakanan ana amfani dashi a cikin masana'antar masana'antu don kauce wa halayen sinadaran da ba'a so.
- Krypton: Ana amfani dashi tare da sauran gas a cikin ƙirƙirar fitilu fitilun filin jirgin sama, saboda tsananin hasken fitilu ja; kuma ana iya amfani dashi a cikin masu shirya silima. Amfani da krypton shima yanada amfani a aikin tiyatar laser.
- Xenon: babban amfani da Xenon shine bayani dalla-dalla na masu fitar da haske tare da halayen kwayar cuta; luminous tubes, hotunan hoto, da kuma a cikin tubes mai kyalli tare da ikon ta da hankalin jan laser.
- Radon: Wannan gas ana samar dashi ne ta sanadiyar lalacewar uranium zuwa radiyo. Saboda wannan kuma saboda yana da tasirin rediyo, yana da karancin aikace-aikace a rayuwar yau da kullun.
Don yin tunani
Kodayake an haɗasu kaɗan wahalar samu a cikin yanayin ƙasa (banda, wataƙila don helium), kuma saboda suna samarwa ko ba da izini ƙalilan halayen tare dasu, iskar gas masu daraja sune mahimmin mahaɗan da zamu iya gani, har ma da amfani dasu a kullun.
Wataƙila amfaninsu ya iyakance ga takamaiman filaye, amma wannan ba yana nufin cewa ba su da amfani kwata-kwata. Daga kunna gidajenmu a cikin kwararan fitila da fitilu, zuwa ajiye abincinmu lokacin amfani dashi a cikin firiji, zuwa ceton rayuka lokacin amfani da maganiWadannan gas din, na dabi'a ko na roba, ba su nuna duk abin da za su iya yi mana ba. Kuma ya tabbata cewa, yayin da bincike ke ci gaba, amfani da shi zai fi girma.
Menene ikon ionize?
da raunin ta