Kwayar halitta ta kunshi kananan kwayoyin da kwayar idanun mutum ba ta iya gani, wadanda ake kira atoms da molecules wadanda sune manyan abubuwan da muka sani a yau azaman kwayar halitta.
Abubuwan da aka ambata ɗazu yawanci shigar da tsarin haɗin da aka sani da haɗin sinadarai, kuma waɗannan ana nazarin su ta hanyar ilmin sunadarai don fahimtar dubban hanyoyin nazarin halittu waɗanda ke faruwa yau da kullun a gabanmu amma ba za a iya saurin fahimta ba. Ta hanyar su ne suka sami damar fahimtar yawancin al'amuran da suka sa duniya ta zama yadda take.
Menene alakar sinadarai?

Duk abubuwan da ake da su a duniya gami da rayayyun halittu, daga cikinsu akwai mutane, sun haɗu da haɗuwa da wasu ƙwayoyin halitta da ƙwayoyin halitta waɗanda suka yanke shawarar shiga ta hanyar aikin da aka sani da haɗin sunadarai. Sanannen abu ne cewa dukkanin kwayoyin halitta da ma wadanda basu da rai (abubuwa marasa rai) suna dauke ne da kwayar halitta, kuma wannan ya dogara da alakar sinadarai don samun damar kirkirar kanta.
Ya danganta da yadda aka hada atam da kwayoyin, zai yuwu a tantance wane nau'in hadin sinadarai ne ake kula da shi, kuma a cikin wadanda suka fi yawa ana samun sinadarin ionic, covalent da na ƙarfe, duk da cewa an sami nau'ikan igiya iri biyu. shaidu waɗanda ba sanannun sanannun lokacin magana game da batun, waɗanda sune haɗin gado na hydrogen da Van der Waals.
Abubuwan haɗin sunadarai ana kiran su waɗancan ƙarfin da ke sanya ƙwayoyi biyu ko sama da haka su zauna tare na wani lokaci, kuma hakan yana ba da izinin watsa wutan lantarki a tsakanin su.
Tsarin jan hankali wanda yake faruwa tsakanin kwayoyin halittar biyu ya juye zuwa wani abu dan baƙon abu, amma idan aka bincika shi da ɗan kulawa, za'a iya fahimtarsa da sauƙi. Babban abin da yakamata a sani shine cibiyoyin da suke da kyawawan zarge-zarge suna kaura, amma a lokaci guda ana iya jan hankalinsu ta hanyar wutan lantarki tare da cajin mara kyau wanda yake akan farfajiyar su, wanda a lokuta da dama na iya fin ƙarfin shi yana sa tsakiya ya motsa.
Lokacin da tsarin haɗin sunadarai yakan faru galibi, idan ba koyaushe ba wasu kwayoyin halitta suna rasa lantarki yayin da wasu ke cin nasara, amma a ƙarshen aikin ana iya kiyaye kwanciyar hankali na lantarki tsakanin duk aikin.
Nau'in nau'ikan 5 na alaƙar sunadarai
Abubuwan haɗin sunadarai da wasu halayensu za'a nuna su a ƙasa don fahimtar yadda suke aiki.

Hanyoyin haɗin karfe
A wannan nau'in haɗin zaka iya ganin yadda aka ƙirƙiri gajimare wanda yake ɗauke da dukkanin kwayoyin halittar, wanda aka samar dashi ta hanyar electrons sako-sako. Ana iya lura da shi a cikin wannan tsari yadda atomatik ke canzawa zuwa electrons da ions, maimakon faruwa kamar yadda ya saba, barin kwayar zarra ta kusa.
Abubuwan ƙarfe na ƙarfe galibi suna samar da cibiyoyin sadarwa waɗanda aka ɗauka a matsayin lu'ulu'u ne, waɗanda suke da mahimmin tsari.
A fuskokin waɗannan cibiyoyin sadarwar zaka iya ganin nau'ikan cibiyoyin sadarwar lu'u-lu'u guda uku, waɗanda suke da maɓallin daidaitawa daban-daban waɗanda suke canzawa dangane da inda suke, kai maki 12, maki 8 kuma na ƙarshe tare da maki 6, ba tare da Duk da haka ba, ana cewa valence matakin karfe atoms ko da yaushe karami.
Ionic bonds
Lokacin da muke magana game da ionic bond, muna so mu koma zuwa ga haɗuwa tsakanin atoms waɗanda ke da ƙananan ƙarfin lantarki tare da waɗanda suke da kuzari iri ɗaya da suka fi na farko girma, waɗanda yawanci ƙarfe ne da wani abu wanda ba ƙarfe ba . Don wannan ya faru ya zama dole daya daga cikin kwayoyin halitta zai iya rasa lantarki, kuma dayan zai iya samun su a jere. Sabili da haka, ana iya bayyana wannan haɗin a matsayin tsari wanda atom biyu suke da jan hankali na lantarki, wanda ɗayan yake shiga tare da jan hankali ɗayan kuma da ƙarancin jan hankali.
An nuna cewa abubuwa wadanda ba karafa ba suna da karancin lantarki a cikin abubuwan da suke hadawa don samun damar zagayawa gaba daya kuma wannan dalilin ne yasa ya zama mai karbar aikin, wanda ake kira anion.
Abubuwan ƙarfe an san su da cations saboda suna da caji mai kyau wanda yake akasin anions, kuma tunda suna da lantarki a ƙarshen abin da suke haɗuwa, suna da ikon haɗawa da wasu ƙwayoyin cuta, a wannan yanayin waɗanda ba ƙarfe ba.
Jagoran abin da aka bayyana, ana iya gane cewa a cikin wannan nau'in haɗin sunadaran atoms suna da ƙarfi ta hanyar ƙarfin lantarki, sabili da haka maɗin yana jawo cation, kuma yana wurin lokacin da za'a iya lura dashi lokacin da ɗayan ƙwayoyin suka samar. yayin da sauran ke sha. Lokacin da wannan mahaɗan ya kasance mai ƙarfi, zai kasance kamar yadda aka bayyana kuma ya daidaita, amma a daidai lokacin da aka sanya shi a cikin yanayi mai ɗumi ko kuma tsoho a cikin wani ruwa, za su sake rabuwa, suna riƙe cajin lantarki.
Vaididdigar haɗin gwiwa
A cikin alaƙar haɗin gwiwa, atoms suna da ikon jan hankali da raba wutar lantarki ko sha su kamar yadda yake a cikin sharuɗɗan da muka ambata a sama, kuma an nuna cewa lokacin da waɗannan suka faru ion sun fi karko.
Kodayake ana iya cewa yawancin hanyoyin haɗin suna da ƙarfin zama masu gudanar da wutar lantarki, amma a wannan yanayin ya nuna cewa babban ɓangare ba. Dukkanin kwayoyin halitta suna hade ne da hadewa, tunda kamar yadda aka ambata a sama yafi kwanciyar hankali.
Waɗannan shaidu suna da nasu rarrabuwa wanda ya banbanta ya danganta ko daɗaɗɗen cakuda ne ko a'a, waɗanda ake kira polar bonds da waɗanda ba a bayyana su ba waɗanda za a ba da taƙaitaccen bayani a ƙasa.
Polar covalent bond
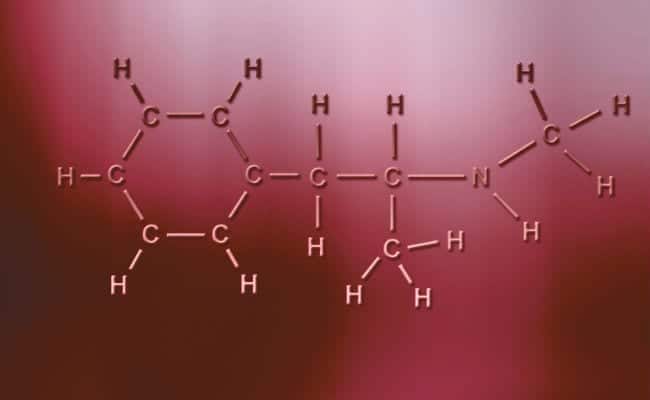
Babban halayyar haɗin haɗin haɗin haɗin polar shine cewa basu da cikakkiyar matsala, a ma'anar cewa atomatik tare da caji mai kyau ko mara kyau na iya samun electrons guda biyu don rabawa ko sarari biyu don sha yayin da ɗayan ke da guda ɗaya, yana mai bambanta lamura. Wadannan suna faruwa kusan kamar ionic bonds amma tare da bambancin da kawai cewa atoms din zasu hada, to hadin polar covalent bond yana faruwa. Don waɗannan su faru dole ne su faru tsakanin abubuwa biyu daban-daban waɗanda ba ƙarfe ba,
Poa'idar haɗin gwiwa
Ba kamar nau'in haɗin sinadaran da aka bayyana a sama ba, a wannan yanayin dole ne a sami atom biyu ko fiye na nau'in da ba na ƙarfe ba. Wannan ya sha bamban da na polar ta kowane fanni, kuma ana iya nuna hakan ta hanyar sanin cewa lokacin da atom biyu na abu daya suke raba wutan lantarki kamar yadda aikin yake gaba daya, sai su kasance daidaito kuma dukkansu suna karba kuma suna ba da lantarki daidai wa daida.
Hydrogen bond bonds
Hydrogen yana da halin kasancewa koyaushe yana da caji mai kyau, kuma don aiwatar da wannan haɗin ya zama dole atom yana jan hankalinsa tare da caji na lantarki, wanda godiya ga wannan aikin yana yiwuwa a lura da yadda ake haɗuwa tsakanin biyu a wanda aka sanya masa suna kamar gadar hydrogen wacce daga inda sunan jarin ya samo asali.
Hanyoyi zuwa Van der Waals
A cikin wannan nau'in hanyoyin, ana iya samun haɗin tsakanin maɓuɓɓugai na dindindin guda biyu, haka kuma tsakanin maɓallan maɓallan biyu, ko kuma akwai yiwuwar yuwuwar samun ƙungiyoyi tsakanin dindindin da wanda aka jawo. Hanya guda daya da hakan zata iya faruwa shine tsakanin kwayoyin halitta guda biyu, wadanda zasu fara aiki lokacin da ake samun jan hankali ko kyama tsakanin kwayoyin ko ta hanyar tsoka mu'amala tsakanin ions da kwayoyin.
Godiya ga karatun da akai ya shafi dukkan nau'ikan nau'ikan haɗin sunadarai na yanzu shi ne cewa ya kasance mai yiwuwa ne a kara fahimtar kadan yadda kwayoyin halitta ke aiki da kuma yadda za a canza shi zuwa wani sabon samfuri ko kuma komawa yadda yake bayan canzawa a cikin aikin musayar lantarki kamar yadda aka bayyana a mafi yawan wadannan matakan.
Duk wannan ilimin an same shi ne sakamakon ci gaban fasaha, tunda a da ana yin hasashe ne kawai game da samuwar kwayar halitta kuma misalin wannan shi ne kasancewar samfuran kwayar zarra na manyan masana falsafa, kodayake ba su da nisa da abin da ke da aka sani a yau, a yau ya kasance mai sauƙi don fahimtar matakai.