Within chemistry, there are countless elements that may or may not be related to each other. There are metals, nonmetals, lanthanides and actinides, transition metals and alkaline earths; and of course we have one of the chemical elements To whom we have paid little attention during chemistry classes, and much less when it comes to moving on with our lives. I am speaking, of course, of noble gases.
These elements that, being so rare in the environment, we cannot analyze too much. Here we will learn about the history of noble gases, their uses and properties, as well as other curiosities. Stay here and learn the coolest things about noble gases.
Let's get to know the gases
They are a group of chemical compounds with very similar properties to each other. For example, under normal conditions they are colorless, odorless, monatomic gases, and have very low chemical reactivity. These are located in group number 18 of the periodic table, and are known as: Helium, neon, Xenon, Argon, Krypton, the radioactive: Radon, and the synthetic: Oganeson.
Its properties can be explained by existing modern theories on atomic structure. Their shell of valent electrons are considered complete, which gives them a limited tendency to participate in chemical reactions, and is one of the reasons why they are poorly understood. In fact, very few noble gas compounds have been prepared to this day.
Where do we get the noble gases?
We get neon, argon, xenon and krypton from the air using fractional distillation and liquefaction methods. Helium is found in natural gas, where it should be typically separated. And radon is obtained through the radioactive decay of compounds dissolved in radium.
And Oganeson is a synthetic element created in 2002, and which obtained its IUPAC nomenclature in 2016. It is known for being quite a reactive element as well as unstable, so not much work has been done with it.
These gases have had very important uses in the fields of lighting, welding and space exploration. Trimix, which is a solution of helium-oxygen-nitrogen, is used in so that divers do not suffer the narcotic effect of nitrogen in the depths. What's more, after knowing the flammability hazards of hydrogen, this was replaced by helium in the creation of airships and hot air balloons.
Properties of these gases
Noble gases get their name from the translation from German edelgas, name used for the first time in 1898 by the chemist Hugo Erdman. With this name sought to refer to the low reactivity rate of these elements. In fact, these are the least reactive elements known, so much so that they are practically inert or non-reactive.
This is because they have a complete valence shell which leaves them with a low capacity to release electrons and makes their behavior close to that of an ideal gas.
In general, noble gases share different properties.
- They are non-metallic elements: Being gases, it does not have any metal particle within its conformation. At the same time they are not capable of reacting with other metals.
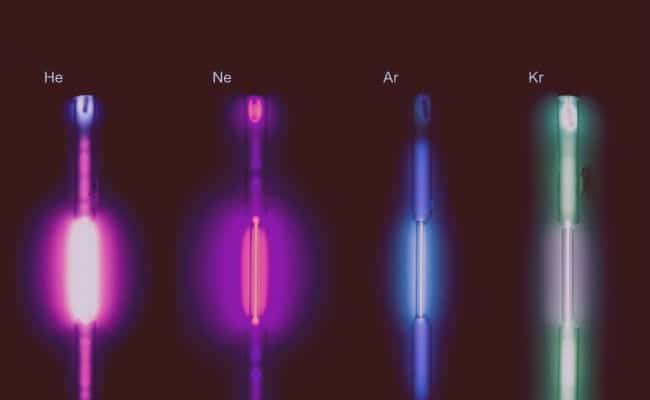
- They are colorless and odorless: although they can be given colors to bulbs and lamps created by means of these gases using electricity, they are originally colorless, and odorless.
- They have a full valence layer: Neon, xenon, argon, krypton, and radon have eight electrons in their last shell. For its part, helium has two electrons. In this way, noble gases have a complete valence shell. That is why, under normal circumstances, these elements do not form links.
- They exist as monatomic gases: As understood, these elements, even the largest atomically, only have one atom.
- They are practically non-reactive: Due to their full valence and their difficulty in delivering electrons, they are considered practically inert.
- They conduct electricity and produce fluorescence: Although very low, these gases are capable of conducting electricity, and in doing so they glow phosphorescently.
- They have a low melting and boiling pointThese noble gases have very low melting and boiling points.
- They have a very low electronegativity: these elements are very low electronegative
- They have a high ionization energy: Your ionization energy is actually the highest in your period.
- They are not flammable: Even due to the flammable cup of hydrogen, it was replaced by helium in the manufacture of airships and balloons.
As with reactivity, their interatomic forces are also very weak, which is why they have low melting and boiling temperatures, and they are all monatomic gases under normal conditions, including gases with a higher atomic mass.
Helium has many properties that no other noble gas or any other element on the periodic table has. His melting point is the lowest in all known ones, besides being the only element that owns a state of superfluidity; a state in which matter is in a liquid state, but can run without losing kinetic energy. Helium needs a pressure of 25atm and a temperature of -272ºC to be able to solidify.
Their full valence shell is also responsible for these gases having a high ionization capacity (the highest in the periodic table). and cannot form ions easily, which shows its stability in its electronic configuration.
The ionization energy decreases as the group decreases, since the atomic radius increases and the valence electrons are further away from the nucleus and therefore less attracted to it. This causes that, although its period is the highest, some noble gases have a comparable ionization energy to that of other elements. For example, the ionization energy of xenon is comparable to the ionization energy of oxygen.
Uses of these gases
By having such low boiling and melting points, they are especially useful in the manufacture of refrigeration equipment, and makes them useful as cryogenic refrigerants as well.
Liquid helium, which boils at 4,2K (-268,93ºC) is used in the manufacture of superconducting magnets, such as those used for magnetic resonance imaging and nuclear magnetic resonance.
Liquid neon, although it does not reach the low temperatures of liquid helium, has more applications in cryogenics, since it has a capacity to 40 times more cooling than liquid helium and 3 times greater than that of liquid hydrogen.
Helium is used as a component of breathable gases to replace nitrogen, thanks to its low solubility in fluids, especially in lipids. Gases are absorbed into the blood and body tissues when there is pressure, such as scuba diving, which produces an anesthetic effect called depth sickness. Due to its low solubility, little helium enters cell membranes, which helps to curb the narcotic effect.
Due to its low combustibility and lightness, and after the Hindenburg disaster of 1937, helium replaced hydrogen in the manufacture of fuel, even despite a loss of buoyancy of 8,6%
These gases are used in lighting due to their conductivity. In the manufacture of incandescent bulbs a mixture of argon and nitrogen is used to fill them. Krypton used in high performance bulbssuch as halogen lamps, which have a higher color temperature and higher efficacy.
Xenon is commonly used in xenon headlights, which, by achieving a light spectrum similar to daylight, is used in film projectors as well as car headlights.
In medicine, helium is used to improve the ease of breathing in asthma patients. Xenon can be used as an anesthetic Due to its high solubility in lipids, which makes it more effective than the usual nitrous oxide, and since it is easily eliminated by the body, it allows a faster recovery.
The acquisition of images that is carried out by means of nuclear magnetic resonance, has xenon combined with other gases. Radon, which is highly radioactive and only available in trace amounts, is used in radiation therapy treatment.
Production and abundance
The abundance and ease with which noble gases can be obtained are in inverse proportion to their atomic number. Therefore, the abundance of these gases decreases as their atomic number increases.
In the universe, helium is the second easiest element to get, after hydrogen, with a mass percentage of approximately 24%. Most of the amount of helium in the universe was formed by primordial nucleosynthesis, but its amount is increasing thanks to the participation of hydrogen in stellar nucleosynthesis (a process that arises by nuclear reactions during the evolutionary process of stars).
The rest of the gases are not nearly as abundant or simple to obtain. Radon, for example, can be form in the lithosphere through alpha decay of radium; Meanwhile he xenon has developed a theory known as the "missing xenon theory" due to its relatively low amount in the atmosphere.
Let's talk a little about each
- Helio: Due to its low combustibility, and because it is the second easiest element to obtain, it has been able to replace hydrogen as the potential element to fill balloons and zeppelins, since they do not explode when they come into contact with fire
- Neon: This gas, due to its fluorescence and its red-orange hue obtained when it comes into contact with electricity, is used for advertising purposes. Easily found in neon lights. Neon tubes and lamps that have other colors are also available, although they actually have other gases inside.
- Argon: This gas is used in incandescent lamps because it does not react with the filament under high temperature and pressure conditions. In fluorescent tubes it generates a green-blue color. It is also used in the industrial field to avoid unwanted chemical reactions.
- Krypton: It is used together with other gases in the creation and manufacture of lamps airport lighting, due to the intensity of the red lights emitted; it can also be used in cinema projectors. The use of krypton is also useful in laser retinal surgery.
- Xenon: the main use of Xenon is the elaboration of light emitters with bactericidal characteristics; luminous tubes, photographic flashes, and also in fluorescent tubes with the ability to excite the ruby laser.
- Radon: This gas is generated by the radioactive decay of uranium to radio. Because of this and because it is very radioactive, it has very few applications in daily life.
To reflect
Although they are composed somewhat difficult to obtain in the natural state (except perhaps for helium), and because they generate or allow rather few reactions with them, noble gases are important compounds that we can see, and even use on a daily basis.
Perhaps their uses are limited to specific fields, but that does not mean that they are completely useless. From lighting our homes in light bulbs and lamps, to keeping our food when used in refrigerators, to save lives when used in medicineThese gases, natural or synthetic, have not yet shown all that they can do for us. And it is certain that, as research progresses, its use will be much greater.
What is the ability to ionize?
and its fragility