One of the great achievements at the scientific level was the classification and organization of the elements. The study of the properties of matter dates back to the time of the alchemists, the scientists of this area always had in mind the importance of establishing a classification system, which would allow the orderly management of the elements that were known at that time. .
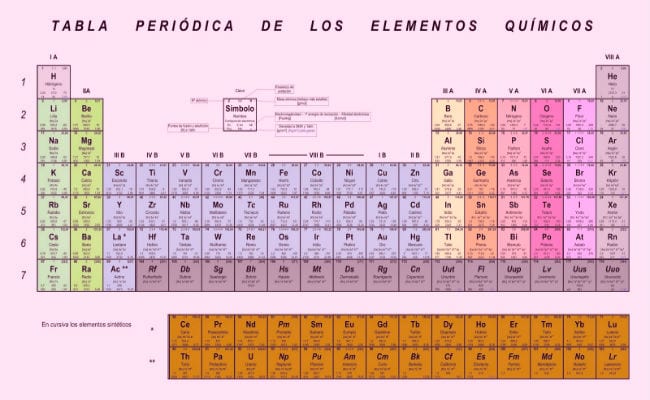
From there, after many attempts, the well-known table of electronegativities was developed, also known as Mendeleev's periodic table, which is the most efficient classification and organization system that we have so far. In it the elements are arranged in function of their electronegativities, which is a measure of the ability of the electrons of its last shell to combine with other atoms, but we will talk about that.
What is electronegativity?
Before going fully into the subject, it is important to clarify that all matter is made up of atoms, as defined by John Dalton in 1803. The atom is the elemental and indivisible unit of matter, which consists of a nucleus, around which electrons and protons rotate in elliptical orbits, and it is the electrons present in the last layer of the element in its state of aggregation that determines the capacity of each material to form compounds. This is what defines electronegativity, the ability of the atom to combine through bonds with other atoms.
This process is defined by the action of two quantities:
- Atomic mass: What is the total mass of protons and neutrons in a single atom.
- Valence electrons: Negatively charged particles located in the last layer of the atom, which constitute the amount of particles available to carry out the exchange in the formation of compounds.
Development of the electronegativity table
In their search for an adequate classification of the elements, many scientists developed ideas around what could be a suitable system, through which the elements could be accessed in an orderly way, taking into account their properties. The following scientists made important contributions that contributed to the development of the current table of electronegativities:
- Antoine Lavoisier: The classification made by this scientist of the elements was carried out arbitrarily, without taking into account any classification criteria, so his classification was not very successful.
- Johann Doberiner: This scientist is known for developing the triads that bear his name. He developed a study in which he grouped elements in a group of three, finding by making comparisons that their relative atomic masses (which are determined using a mass spectrometer), and certain values of their physical properties, were related to each other. Therefore, they could be predicted by means of mathematical approximations. British chemist John newlands, worked on the basis developed by Dobereiner, and thus managed to order the elements in a table with groupings of elements of relative atomic masses in increasing form; With this grouping, the British sought to develop a table where a pattern of periodic repetitions of the physical properties of the elements. Since such repetitions were grouped around 8 elements, they were denoted by the name of "Law of octaves".
- Lothar Mayer: He is known for expanding knowledge in the field of studying the relationship of physical properties and atomic properties of components. His work was complementary to, and in turn independent from, the work produced by Mendeleev.
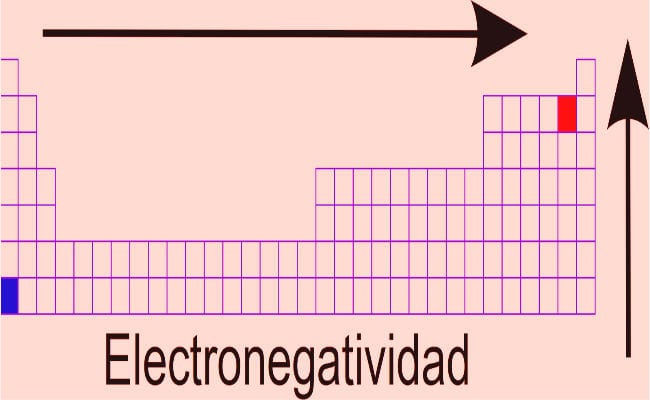
- Dmitri Mendeleev: Based on the postulates of the periodic law, this scientist developed the most successful element classification work, which is still in force (with modifications, in which the new discovered elements have been added. He classified the elements taking into account their electronegativities, and had the vision of leaving boxes where no element fit, anticipating that there would fit an element that had not yet been discovered. Known elements that escaped order parameters were noted separately, instead of being included arbitrarily (mistake made by Lavoisier and Newlands). Regarding the electronegativity within the table, the general rule is: Electronegativity is a value that increases as we move to the right of the table, observing a decrease when moving to the left. The elements at the top of the table have higher electronegativity values.
Electronegativity scales
The different values of electronegativity determine the type of bond formed, therefore, the study of this process was the object of interest, and two postulates were developed:
Pauling scale: According to Pauling's studies, it was established that electronegativity is a variable property, since it depends on the oxidation state of the element. His observations allowed him to determine that, if a subtraction, or difference, of the electronegativities was made, we could predict the type of bond that would be formed, since he established a scale:
- Ionic bond: Electronegativity gradient greater than or equal to 1.7. This bond usually occurs between metallic and non-metallic elements.
- Covalent bond: When the difference is in the range of 1.7 to 0.4. It is common to see them in non-metallic compounds.
- Polar link: For differences equal to or less than 0.4.
Mulliken scale: It is based on the electronic affinity of the elements, which defines their tendency to acquire a negative charge, which is what defines an element's ability to accept electrons. It also works with ionic potentials, which in turn determine the element's predisposition to take on a positive charge (positively charged elements are those that donate electrons from their last shell). This scale works with average values.