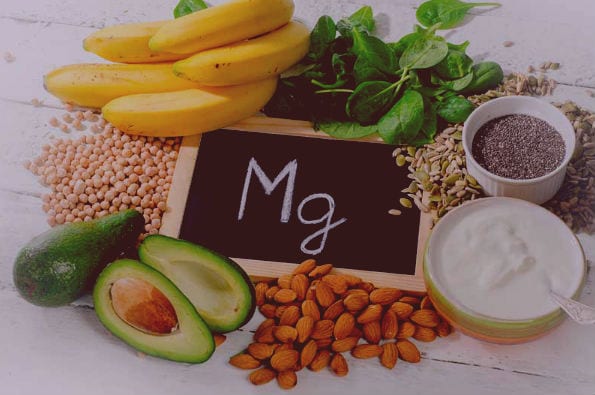Within our world, there are numerous elements that make up the matter we know today. For centuries, little by little, names have been known for many of them. When we speak of elements we refer to all those chemical materials that we find around the world.
Of those microscopic elements that we can find and that are often the ones that regulate the life that we know. Some as well known and necessary as oxygen, and others that we may overlook from time to time, such as noble gases. However, common or not, all of them have a function on the planet, and it is important that we know them.
If we take an example, magnesium may be a chemical element that we take for granted very often, but like most elements, it serves a very important, and in many cases, a definitive function.
In this case, we can find it commonly, in our body, where it not only has the task of fulfilling one, but several functions that can be beneficial for us as human beings. In this post we will delve into the periodic table, and discover how important an element so essential, but so little valued, can have for us.
Let's talk a little about this element
In a general way, magnesium is the chemical element that, in the periodic table, we know by its acronym Mg; we know the atomic number, which is 12, and its atomic weight is 24,305u. It is known as the seventh most abundant element in the earth's crust, and the third most abundant dissolved in water worldwide. The magnesium ion is very important and essential for all living cells. The pure metal is not found in nature. Once produced through magnesium salts, this metal can be used as an alloying element.
On a medical level, it is very important to man. This macromineral is present in bones. At the same time, it has a regulatory function, since it is involved in many tasks of obtaining energy at the cellular level.
It plays a very important role in our body to put energy into metabolism, and it is particularly important in physically active people. This mineral also intercedes in the nervous system and plays a basic role in muscle work. This mineral helps to relax the muscles, and therefore guarantees a good functioning of the muscular system. Also in the cardiovascular.
Magnesium and its history
When we talk about the term we use today, or its etymology, we discover that its name is generated from Thessaly, a region in the Prefecture of Magnesia. It is related to magnetite, and also manganese, which were named for this same area.
In the XNUMXth century, a farmer from Epsom, England, took his cows to drink water from a trough. However, the animals refused to drink, due to the bitter taste of the local water. The farmer, however, discovered that the water managed to heal skin scratches and rashes. Over time, the substance became known by the name of Epsom salts and its fame spread far and wide. The substance was later recognized as magnesium sulfate.
In 1755, the Englishman Joseph Black recognized hydrated magnesium as a chemical element, while the metal itself was produced by Sir Humphry Davy in England in 1808.
What are their characteristics?
The mineral magnesium is not found in nature as a metal, but is part of various compounds, whether they are metals, oxides or salts. It is a light metal and is insoluble; moderately strong and silvery in color.
This element is covered by a thin layer of oxide, and due to this it does not need to be stored in oxygen-free environments, as other alkali metals do. However, when in contact with this element, it becomes less lustrous; this being the only visible disorder.
Like its lower neighbor on the periodic table, calcium, this element reacts with water at room temperature, although much slower. When immersed in water, small hydrogen bubbles form that rise to the surface, although if it is sprayed it reacts more quickly.
It also reacts with hydrochloric acid, producing heat and hydrogen which, like water, is released in small bubbles. This reaction occurs faster at high temperatures.
It is a highly flammable metal, which it ignites much more easily if we find it in the form of chips or dust. As a solid mass, it takes much less time to fully ignite. When burned, it creates an incandescent white flame, and for a long time it was used in photography; initially as flammable magnesium powder, and then magnesium strips present in electric flash bulbs.
Known uses
- The known compounds of magnesium, mainly its oxide, are used as a refractory material in furnaces for the production of steel, iron, non-ferrous metals, cement and glass. It can also be used in agriculture, chemical and construction industries.
- Its main use is in alloys with aluminum, creating aluminum-magnesium alloys that we can find in beverage containers. Aluminum alloy, especially the aforementioned aluminum-magnesium alloy, is used in automobile components such as wheels and various machinery.
- It is an excellent additive in conventional propellants.
- It is a reducing agent in obtaining uranium and other metals from their salts.
- Magnesium carbonate can be seen in gymnastics and weightlifting sporting events, as it is essential when it comes to improving the grip of objects.
- Milk of magnesia, magnesium chloride, magnesium sulfate (Epsom salts), and magnesium citrate they have very varied uses in medicine.
Magnesium for health
Within the human body, the mineral magnesium, and many of its compound forms have great applications when it comes to improving our health. As we mentioned before, within the body, this element can perform multiple functions.
- It can be involved in maintaining healthy teeth, heart, and bones.
- Helps with the formation of proteins
- It is an important part of the bone structure, as it is found in bones and muscles as frequently as calcium.
- It is involved in nervous contraction and nerve transmission.
- It participates in energy metabolism, in the release of enzymes that generate glucose.
Where is this mineral found
To find it so that it can be ingested, we can find it in different foods.
- Vegetables
- Vegetables
- Whole grain foods
- Seeds and nuts
- We can even find it in dairy products, chocolate, meats (to a lesser extent) and coffee.
We can easily find it in these elements because, being a mineral, it easily adheres to the earth, and when planting in it the vegetables that grow in it will contain a level of magnesium comparable to that found in said soil. That is why in meat they are obtained to a lesser extent, since in animals, magnesium is already digested and trapped in their cells, and not in a more natural way.
Magnesium deficiency?
There is no conclusive evidence that tells you the level of magnesium itself in the tissues, and that can tell you what the optimal levels of this are in the body. However, there are symptoms that can tell you if you are experiencing a magnesium deficiency. These are:
- Loss of appetite
- Nausea and vomiting
- Headaches
- Weakness and fatigue
Magnesium, finally, is one of the elements that exist that, although many times we do not pay attention to it, it is the most important for our way of life, since it is not only needed to carry out various bodily functions, but it is also needed to the elaboration of materials for daily use, and fulfills its own function in nature, because when it is in the ground it is useful for plants, as well as for us who consume them.
Perhaps before we did not have much knowledge of this mineral, but today, knowledge is power, and we can discover important things about this element day by day.