Matter is made up of small particles that are invisible to the human eye, which were called atoms and molecules that are the main components of what we know today as matter.
The aforementioned particles are usually enter a bonding process known as chemical bonding, and these are studied by chemistry in order to understand the thousands of biological processes that occur daily in front of us but that cannot be easily perceived. It is through them that they have managed to understand most of the events that make the world the way it is.
What are chemical bonds?

All existing things in the world including living beings, among which are human beings, are made up of the union of some atoms and molecules that decide to join through a process known as a chemical bond. It is well known that all living organisms and even inert ones (inanimate objects) are made up of matter, and this depends on chemical bonds to be able to create itself.
Depending on how the atoms and molecules are joined, it is possible to determine what type of chemical bond is being treated, and among the most common ones can be found ionic, covalent and metallic bonds, although two types of bonds have been found. new bonds that are not very well known when it comes to the subject, which are hydrogen bridge bonds and Van der Waals.
Chemical bonds are called those forces that make two or more atoms stay together for a certain time, and that allow the transmission of electrons between them.
The process of attraction that occurs between the two atoms in something a little strange but that if analyzed with a little attention can be understood very easily. The main thing to know is that the nuclei that have positive charges move away, but at the same time they can be attracted thanks to the negatively charged electrons that are on their surface, which on several occasions can be greater than the force that it causes the nuclei to move away.
When the chemical bonding process happens usually, if not all the time some atoms lose electrons while others are winning, but at the end of the process an electrical stability can be observed among all the action.
The 5 types of chemical bonds
The chemical bonds and some of their characteristics will be shown below in order to understand how they work.

Metal links
In this type of bond you can see how a cloud is created that holds the entire set of atoms together, which is formed by the loose electrons. It can be observed in this process how the atoms are transformed into electrons and ions, instead of happening as it would normally be, leaving an adjacent atom.
Metallic bonds usually form networks considered crystalline, which have a high coordination index.
On the faces of these networks you can see three different types of crystalline networks, which have different coordination points that change depending on where they are, reaching 12 points, 8 points and the last with 6 points, without However, it is said that the valence level of metal atoms is always small.
Ionic bonds
When we are talking about ionic bonds, we want to refer to the union between atoms that have little electrostatic energy with those that have an energy of the same type greater than the first ones, which are usually a metallic element and a non-metallic element. For this to happen it is necessary that one of the atoms can lose electrons, and that the other can gain them consecutively. Therefore, this bond can be described as a process in which two atoms have an electrostatic attraction, in which one participates with greater attraction and the other with less attraction.
It was shown that non-metallic elements lack an electron in their composition to be able to have their complete orbit and it is for this reason that it becomes a receiver of the process, which is called an anion.
Metallic elements are known as cations because they have a positive charge that is the opposite of anions, and since they have an electron in the last of their composition, they have the ability to bind to other atoms, in this case non-metallic ones.
Guided by what has been described, it can be deduced that in this type of chemical bond the atoms are attracted by an electrostatic force, and therefore the anion attracts the cation, and it is there when it can be observed when one of the atoms yields while the other absorbs. When this compound remains solid, it remains as described and stable, but at the precise moment it is placed in a humid environment or by default in some liquid, they would separate again, maintaining their electrical charges.
Covalent bonds
In covalent bonds, atoms have the ability to attract and share electrons or absorb them as in the cases mentioned above, and it has been shown that when these occur the ions are much more stable.
Although it can be said that most of the links have a capacity to be conductors of electricity, but in this case it turns out that a large part is not. All organic matter is made up of covalent bonds, since as mentioned above it is much more stable.
These bonds have their own division that varies depending on whether it is a pure mixture or not, which have been called polar bonds and the nonpolar ones of which a brief explanation will be given below.
Polar covalent bond
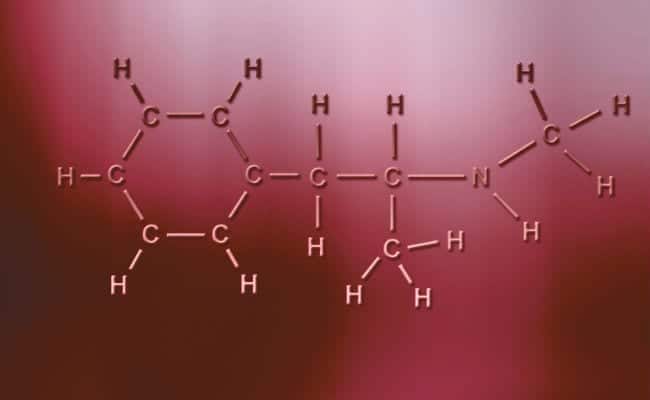
The main characteristic of polar covalent bonds is that they are totally asymmetric, in the sense that atoms with a positive or negative charge can have two electrons to share or two spaces to absorb while the other only has one, varying the cases. These happen practically the same as ionic bonds but with the only difference that for the atoms to unite, a polar covalent bond occurs. For these to occur they have to occur between two totally different non-metallic elements,
Nonpolar covalent bond
Unlike the type of chemical bond described above, in this case there must be two or more atoms of a nonmetal of the same type. This is totally different from polar in every way, and this can be demonstrated by knowing that when two atoms of the same element share electrons as the process is totally symmetric, they remain balanced and both receive and donate electrons equally.
Hydrogen bond bonds
Hydrogen is characterized by always having a positive charge, and in order to carry out this bond it is necessary that it be attracted by an atom with an electronegative charge, which thanks to this process it is possible to observe how a union is formed between the two at the one that was denominated like a hydrogen bridge that is from where the name of the bond derives.
Links to Van der Waals
In this type of links, the union between two permanent dipoles can be found, as well as between two induced dipoles, or there may be the possibility that unions will be found between a permanent and an induced dipole. The only way for this to happen is between two symmetric molecules, which begin to act when there is an attraction or repulsion between molecules or by default the interaction between ions and molecules.
Thanks to the constant study that applies to all types of existing chemical bonds is that it has been possible to understand a little more how matter works and how it can be transformed into a totally new product or return to its shape after changing in an electron exchange action as described in most of these processes.
All this knowledge has been achieved thanks to the advancement of technology, since previously it was only speculated about the existence of atoms and an example of this is the existence of the atomic models of great philosophical thinkers, although they were not so far from what is known today, today it has been possible to better understand the processes.